Medion has long prioritized excellence and quality in the production of its products, a commitment that is clearly reflected in the achievement of Good Manufacturing Practice (GMP) certification, both at the national and international levels.
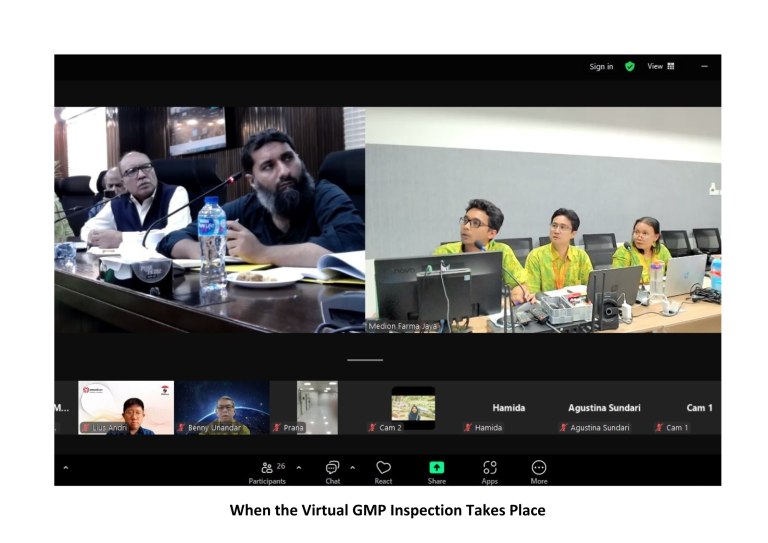
On October 31st, 2024, Medion marked a significant milestone by conducting its inaugural Virtual GMP Inspection from Pakistan. This inspection, organized by the Drug Regulatory Authority of Pakistan (DRAP), was a critical part of the re-certification process for Medion’s GMP certification, a prerequisite for the registration of products in Pakistan.
The event commenced with an introduction to Medion’s personnel and company profile by Andri Yulius, Assistant Manager for International Markets, and Apt. Agung Setiawan, S. Farm, Assistant Manager of Biological Product Quality Assurance. This was followed by a virtual plant tour, conducted via live camera feed, which provided an in-depth view of the vaccine production process—from raw material preparation to the storage of finished products in the warehouse. The session concluded with a Q&A segment with the inspectors.
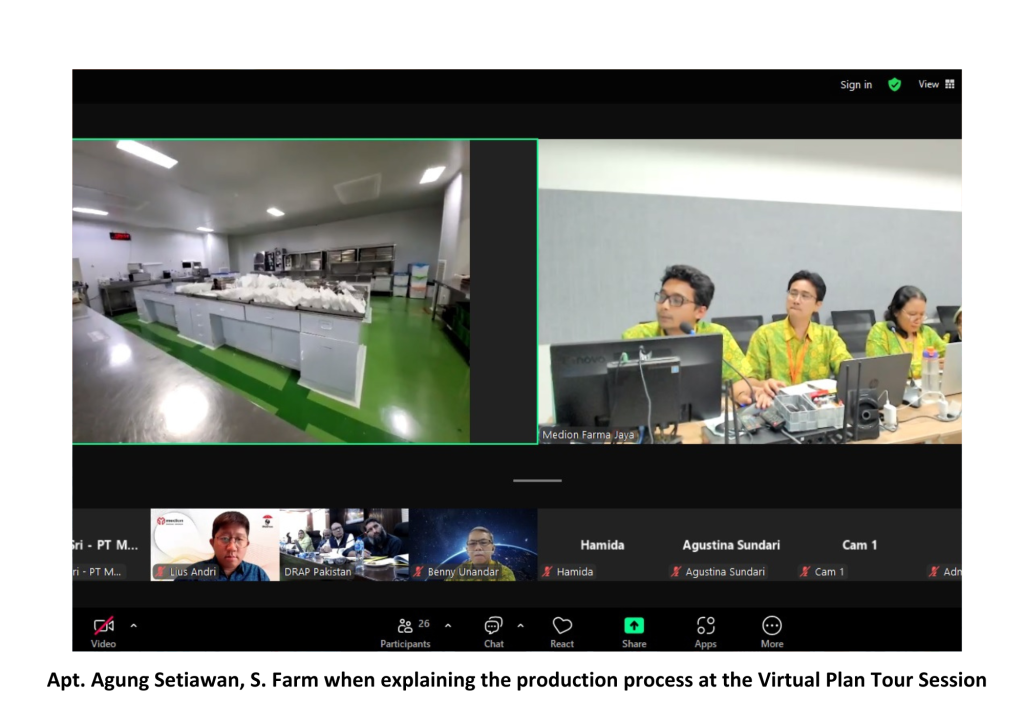
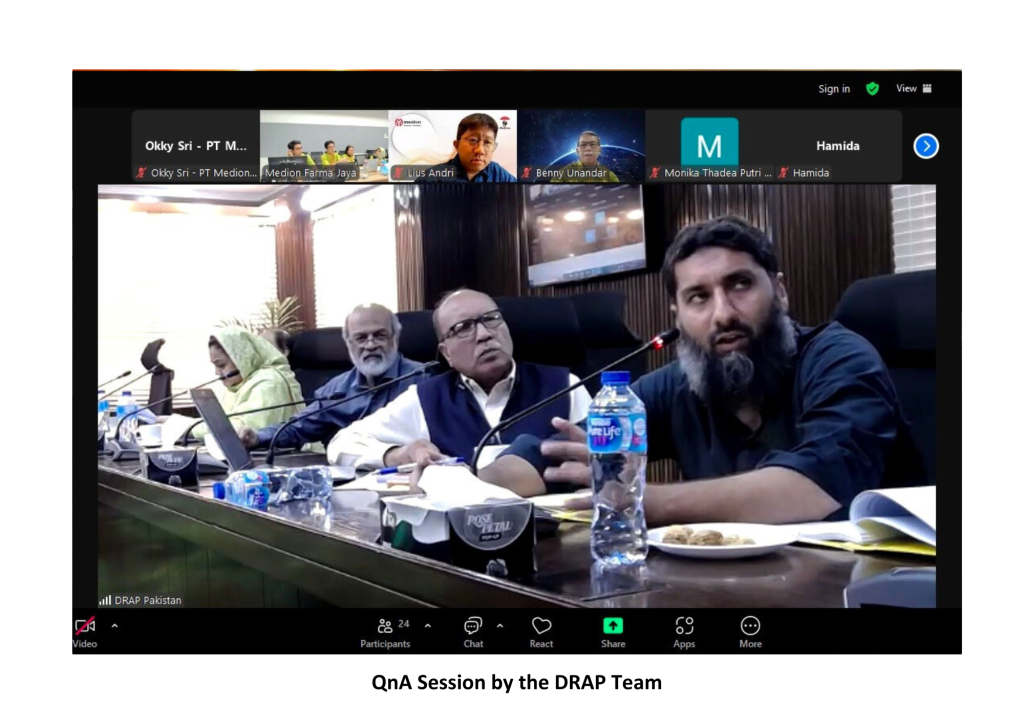
The inspection results indicated that Medion’s vaccine production facilities fully comply with the stringent requirements outlined in GMP standards. The DRAP team, represented by Dr. Qurban Ali, Expert Member of the Registration Board, and Mr. Muhammad Zubair Masood, Deputy Director (PE&R) of DRAP, expressed their satisfaction with the high standards of Medion’s production facilities.
Medion extends its sincere gratitude for the positive feedback and recognition received. The successful application of this technology in conducting the virtual GMP inspection opens up new opportunities for Medion, positioning the company for further business expansion. This achievement underscores Medion’s capability to conduct high-quality virtual inspections, and the company remains firmly committed to delivering a comprehensive range of top-tier vaccines, ensuring its competitive edge in the global market.
